John JO Accarino MD, 1,2, Timothy G Chow MD, 3, Allison Ramsey MD, 4,5, Christine RF Rukasin MD, 6,7, Alexei Gonzalez-Estrada MD, 7, Anne Y Liu MD, 8, David A Khan MD, 3, Kimberly G Blumenthal MD, MSc, 1,2, USDAR Study Team
Date Published
December 2024
Affiliations
- Division of Rheumatology, Allergy, and Immunology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
- Harvard Medical School, Boston, MA, USA
- Division of Allergy & Immunology, Departments of Pediatrics and Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA
- Rochester Regional Health, Rochester, NY, USA
- Department of Allergy/Immunology, University of Rochester School of Medicine and Dentistry, Rochester, NY
- Division of Allergy and Immunology, Phoenix Children’s Hospital, Phoenix, AZ
- Division of Allergy, Asthma and Immunology, Mayo Clinic Arizona, Scottsdale, AZ
- Division of Allergy, Immunology and Rheumatology, Department of Pediatrics, and Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, CA
Full Article
Abstract
Pediatric antibiotic labels are common, and unnecessary antibiotic avoidance is associated with negative personal and public health outcomes; as a result, there is an increasing emphasis on the importance of pediatric antibiotic allergy evaluations. Established consensus testing protocols are lacking.
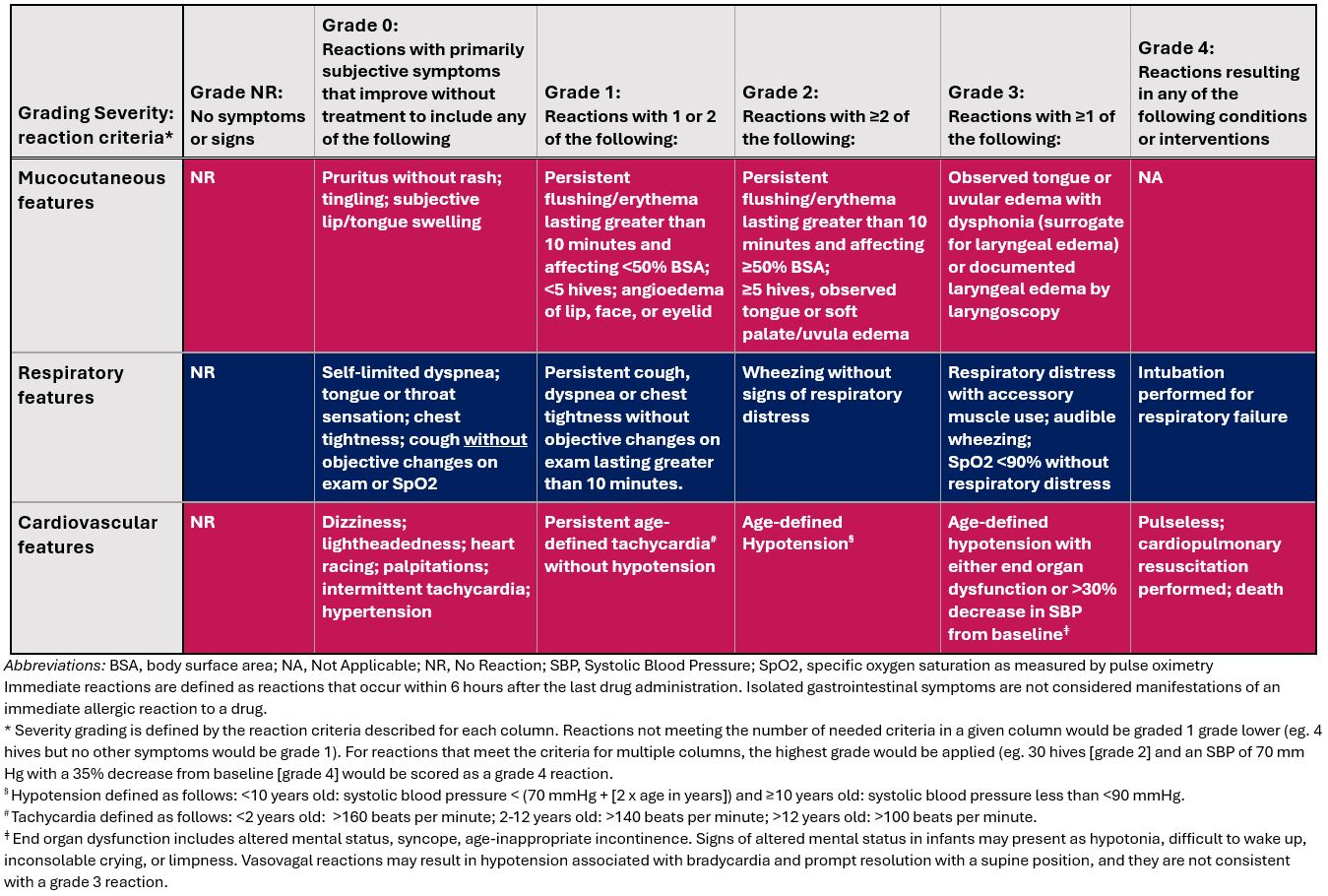
This rostrum aims to provide a rationale and framework for standardization for pediatric antibiotic allergy protocols and assessment of positive reactions through a pediatric-specific adaptation of the USDAR immediate reaction grading scale to create consistency for multi-site research collaboration efforts such as USDAR-Peds
The United States Drug Allergy Registry Pediatrics (USDAR-Peds) is a multi-site prospective study designed for epidemiology and outcome evaluations of pediatric drug hypersensitivity reactions (HSRs). Consensus meetings establishing the contents of this rostrum were performed, on average, bimonthly from February 2023 through September 2024 by allergy specialists with consistent exposure to pediatric drug allergy evaluations. In between meetings, iterations of this unified approach were made based on authors’ clinical experience with any differences resolved by literature review of best available data regarding the dosing of challenges, utility of skin testing, agents tested, timing of observation, and grading of symptoms.
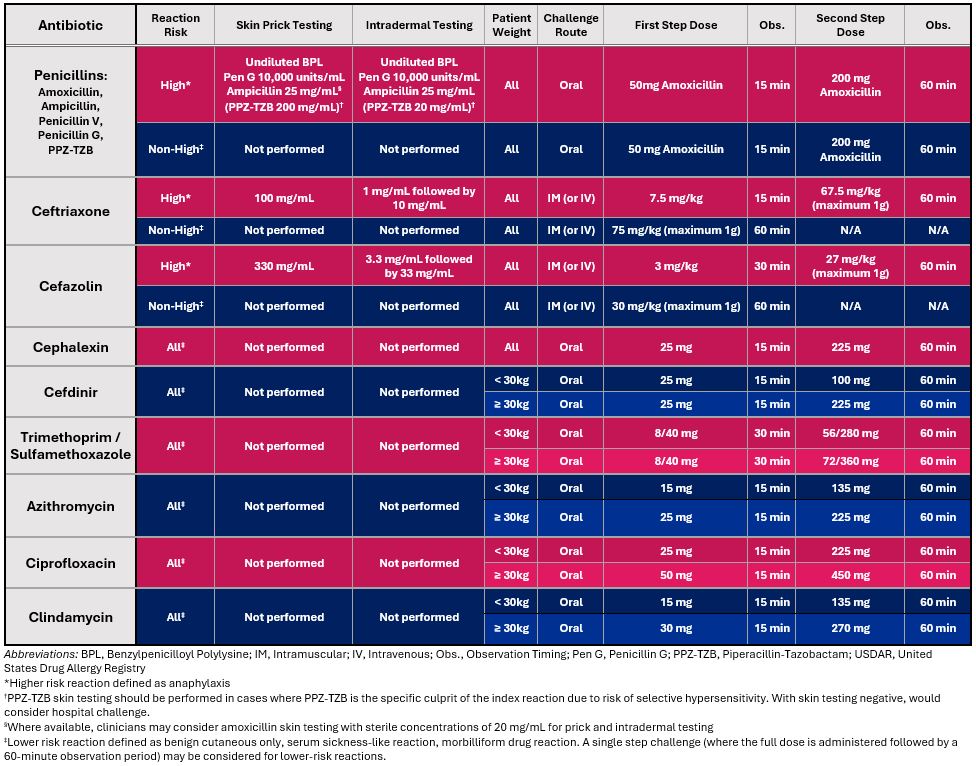
Tables 1 and 2 outline the USDAR-Peds diagnostic testing approach and reaction severity grading scale for immediate drug reactions, respectively. Persons of all ages can be safely evaluated for drug allergies. If the clinical benefit of drug allergy evaluation outweighs the expected risks, even infants can undergo assessments including direct oral challenge testing. For penicillin allergy, most children can be classified as low-risk and safely undergo direct oral challenge. Confirmed anaphylaxis to penicillins is rare in children, and data are limited on the optimal approach to these cases, highlighting the need for further investigation through multi-site prospective studies. In the rare cases of confirmed anaphylaxis, the authors consider PST followed by a DC as the most prudent course. Skin prick testing, intradermal testing, and challenges are indicated based on reaction history characteristics. The increased rate of confirmed allergy with parenteral cephalosporins also led to author consensus on the utility of skin testing and intradermal testing in patients with high-risk (i.e., anaphylactic) immediate index reactions to parenteral cephalosporins. For the vast majority of non-high risk parenteral cephalosporin reaction histories, a single step full dose challenge suffices. For antimicrobial sulfonamides, most hypersensitivity reactions are delayed cutaneous reactions; skin testing has limited value. For macrolides, fluoroquinolones, and clindamycin, skin testing is not proposed as a component of routine evaluation for these agents. For non-severe reactions, a direct challenge is proposed, and consistent with approaches in adults.
Appropriate antibiotic allergy evaluations in children can result in improved antimicrobial stewardship, reduced healthcare costs, and improved long-term patient health. USDAR-Peds provides a framework for a uniform approach across a pediatric drug allergy research network towards pediatric antibiotic allergy protocols and assessment of positive reactions to create consistency for multi-site research collaboration efforts.